How It Works:
Chemistry is the driving force behind the operation of batteries. A battery is composed of one or more galvanic (voltaic) cells, which produce and store electricity through chemical reactions.
- A galvanic cell consists of two half-cells: an oxidation cell and a reduction cell.
- Each half-cell includes an electrode and an electrolyte. Ions derived from the electrode undergo oxidation or reduction reactions, releasing or accepting electrons.
- The chemical energy is converted into electrical energy as electrons flow through an external circuit.
Redox Reactions:
- Oxidation: Loss of electrons (LEO – Loss of Electrons is Oxidation)
- Reduction: Gain of electrons (GER – Gain of Electrons is Reduction)
Oxidation and reduction reactions must occur together in a redox reaction. The substance that loses electrons is called the reducing agent (reductant), and the one that gains electrons is the oxidizing agent (oxidant).
Oxidant + ne⁻ → Reductant
Reductant → Oxidant + ne⁻
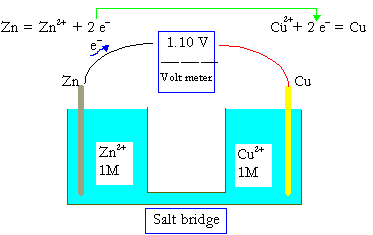
In a Copper-Zinc Voltaic Cell:
- Zinc (Zn) serves as the anode and undergoes oxidation: Zn → Zn²⁺ + 2e⁻
- Copper (Cu) serves as the cathode and undergoes reduction: Cu²⁺ + 2e⁻ → Cu
Educational Benefits:
- Demonstrates fundamental concepts of oxidation, reduction, and electricity generation
- Excellent for science fairs, chemistry lessons, and interactive learning
- Reinforces understanding of redox reactions and galvanic cells
Specifications:
- Copper Plate Size: 10 x 40 mm
- Thickness: 0.3 mm
Illustration:
How It Works:
Chemistry is the driving force behind the operation of batteries. A battery is composed of one or more galvanic (voltaic) cells, which produce and store electricity through chemical reactions.
- A galvanic cell consists of two half-cells: an oxidation cell and a reduction cell.
- Each half-cell includes an electrode and an electrolyte. Ions derived from the electrode undergo oxidation or reduction reactions, releasing or accepting electrons.
- The chemical energy is converted into electrical energy as electrons flow through an external circuit.
Redox Reactions:
- Oxidation: Loss of electrons (LEO – Loss of Electrons is Oxidation)
- Reduction: Gain of electrons (GER – Gain of Electrons is Reduction)
Oxidation and reduction reactions must occur together in a redox reaction. The substance that loses electrons is called the reducing agent (reductant), and the one that gains electrons is the oxidizing agent (oxidant).
Oxidant + ne⁻ → Reductant
Reductant → Oxidant + ne⁻
In a Copper-Zinc Voltaic Cell:
- Zinc (Zn) serves as the anode and undergoes oxidation: Zn → Zn²⁺ + 2e⁻
- Copper (Cu) serves as the cathode and undergoes reduction: Cu²⁺ + 2e⁻ → Cu
Educational Benefits:
- Demonstrates fundamental concepts of oxidation, reduction, and electricity generation
- Excellent for science fairs, chemistry lessons, and interactive learning
- Reinforces understanding of redox reactions and galvanic cells
Specifications:
- Copper Plate Size: 10 x 40 mm
- Thickness: 0.3 mm
Illustration: